Analyze. Design. Optimize.
Pharmacokinetic (PK) modeling is a powerful and cost-effective tool for developing, optimizing and de-risking clinical trial designs.
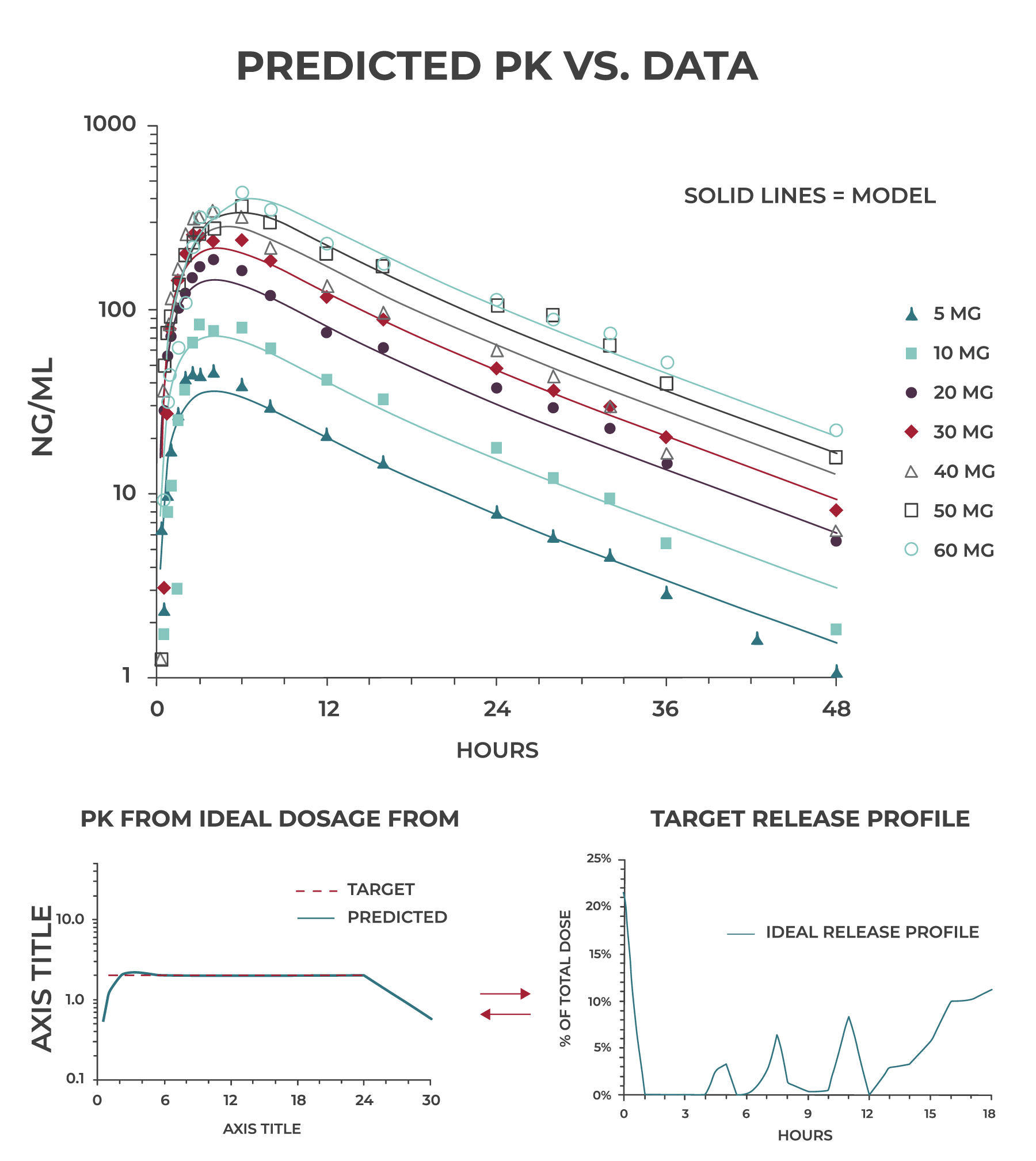
Our team of pharmacokineticists/modelers use sophisticated software such as WinNonLin® and GastroPlus® as well as internally developed tools to generate multi-compartmental predictive models of in vivo drug release, absorption, distribution, metabolism, and excretion.
The goals of these models are to:
- Predict clinical outcomes from animal data that go beyond allometric scaling
- Assess outcomes of different dosing regimens
- Identify the dosing regimen most likely to provide the desired plasma concentration profile
- Predict outcomes with other release profiles, dosing regimens, and even in different species
Determining the Optimal Dosing Regimen
When we observe non-linear PK, very rapid clearance or significant accumulation in preclinical or early clinical studies, we use predictive PK modeling to evaluate dosing paradigms and determine what is most likely to work and what isn’t. This goes a long way toward de-risking the program and may result in significant cost and time savings.
Designing CR Dosage Forms
Zero-order release is not always the ideal profile for a CR dosage form. With predictive PK modeling, we work backward from the target plasma profile to generate an ideal release profile that becomes our target for formulation development.
Assessing Dosage Form Feasibility
Will an enteric-coated tablet do the trick? Is a controlled release product even feasible? Predictive PK modeling is an excellent tool to assess feasibility with relatively little time or cost outlay.
Intellectual Property Generation
Predictive PK modeling is especially effective in the generation of protective IP. In many cases, the ideal release profile for a dosage form is not immediately obvious. That said, once we determine what it is, we can help prepare a patent that covers not just one CR dosage form, but also multiple formulation approaches used to meet the target.