We advance drugs on their path to and through the clinic.
Full-spectrum services and expertise that span nearly every niche in pharmaceutical development.
Discovery support, formulation, CMC, DMPK, toxicology, regulatory, non-clinical development, early clinical development, strategic leadership, global project management. All on your terms. All on your timeline and budget. A vast number of moving parts are involved in getting a new therapy into the clinic, and an even larger number are involved in getting it from First-in-Man to pharmacy shelves. At PharmaDirections, we provide expertise coupled with project leadership and management to help you find the fastest, most strategic and cost-effective path from late discovery to clinical proof-of-concept to approval.
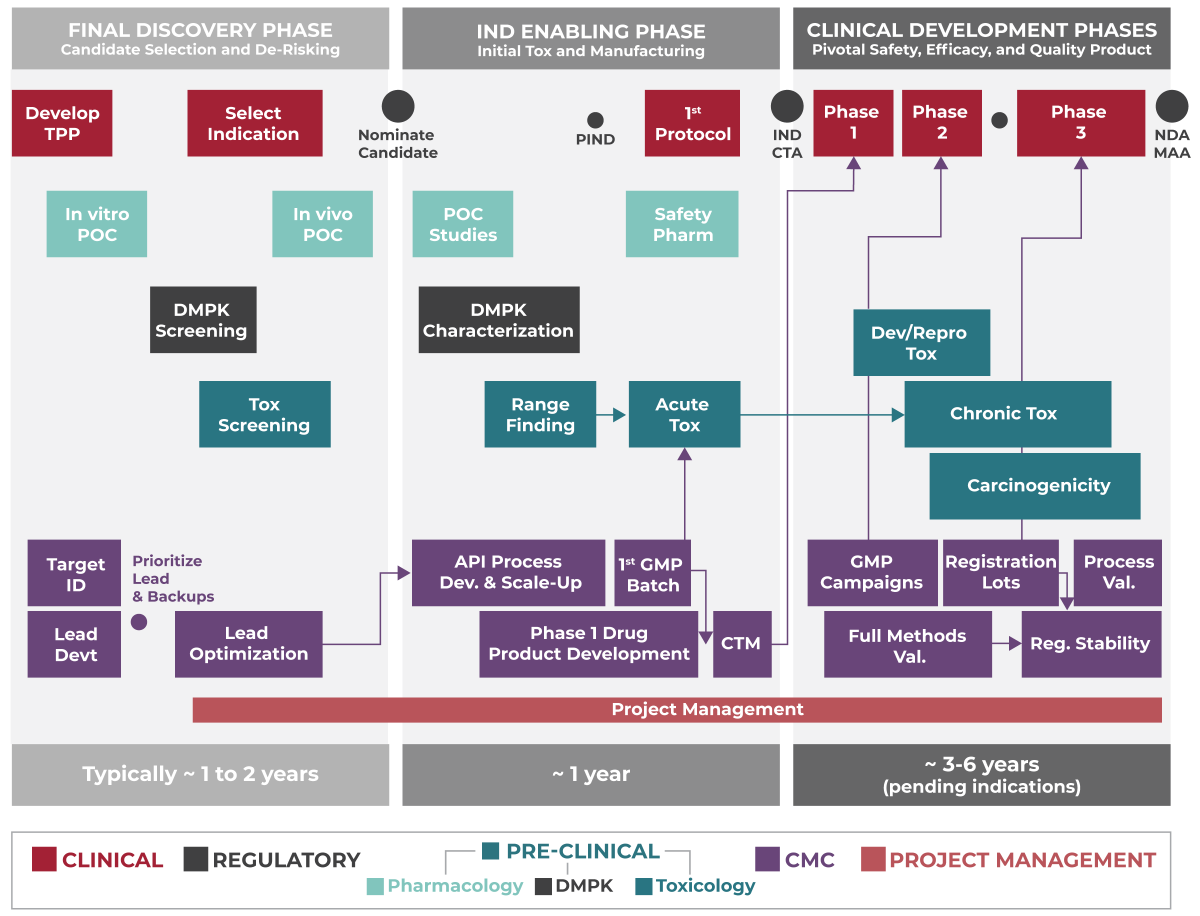
OUR PROCESS
Each molecule is different and warrants its own customized approach. Yet the diagram below represents a typical engagement and journey.
BIG THINKING. PROVEN EXPERIENCE.
Providing a More Sophisticated Path Through Drug Development
We are the hands-on virtual drug development partner for small, start-up and micro-biotech companies who want to bring innovative concepts and compounds to market and enhance people’s lives.
